Health
Oxford-AstraZeneca vaccine gets emergency use approval in Nepal
The decision comes at a time when Foreign Minister Gyawali is in India on a three-day visit.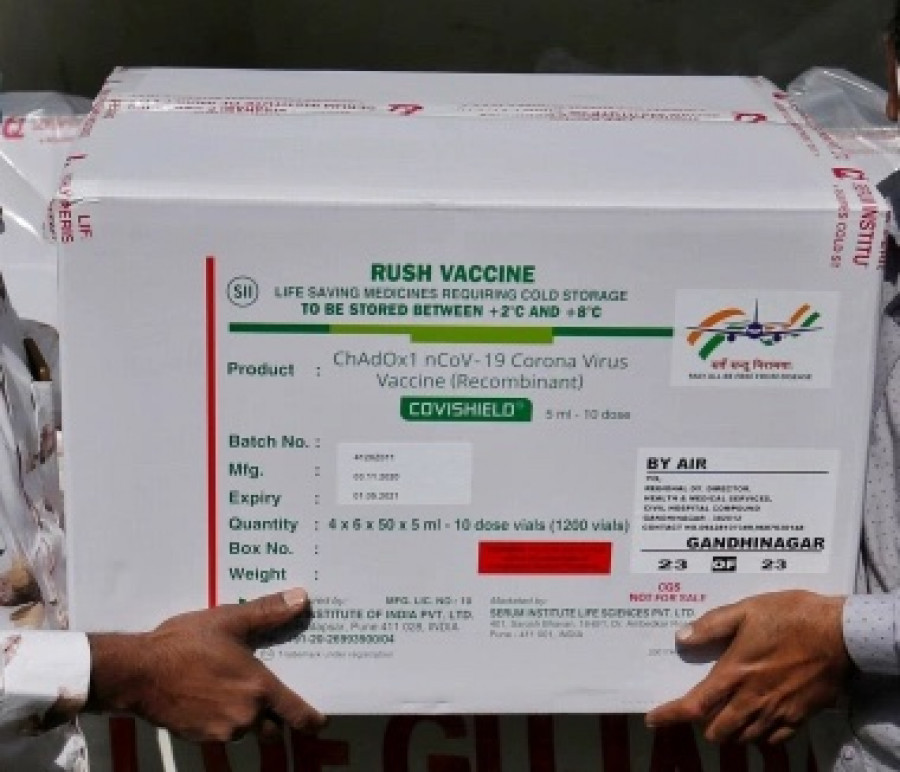
Arjun Poudel
Nepal’s drug regulator on Friday granted emergency use approval to a Covid-19 vaccine developed by the University of Oxford and pharmaceutical giant AstraZeneca and manufactured in India.
The Department of Drug Administration’s decided to grant emergency use authorisation to the Covishield vaccine being manufactured by the Pune-based Serum Institute of India, on Friday, the department said in a statement.
[Read: Don’t rely on one country for Covid-19 vaccines, experts say]
“We have given emergency use approval to Covishield,” Bharat Bhattarai, director-general at the department, told the Post. “Now the Covishield vaccine can be imported and administered in Nepal.”
Emergency use authorisation (EUA) is granted for some drugs and vaccines by authorities such as the US Food and Drug Administration during a declared emergency when officials can make a judgment that the drug is worth releasing—even without all the evidence that would fully establish its effectiveness and safety. Such a decision is taken when there’s enough evidence to suggest that patients have benefited from the drug/ vaccine.
On Wednesday, the department had issued a notice calling on manufacturers of vaccines listed by the World Health Organization or their authorised agents to apply for emergency use authorisation at the earliest.
At least three vaccine manufacturers—two of them Indian and one Chinese— responded to the call and applied for emergency use approval in Nepal, according to Bhattarai. While Serum Institute’s application was approved on Friday, applications from the Hyderabad-based Bharat Biotech and the Beijing-based Sinopharm are being reviewed.
[Read: Money should be no issue for government to procure Covid-19 vaccines, experts say]
India issued its emergency use approval for Covishield and Bharat Biotech’s Covaxin on January 3.
Neighbouring Bangladesh, which pre-ordered 30 million doses of the vaccine, gave its nod to Covishield on January 7.
The decision comes at a time when Nepal’s Foreign Minister Pradeep Gyawali is in India on a three-day visit. Nepali officials are hopeful that a government-to-government deal could be signed for the supply of vaccines during the visit.
The DDA’s announcement also comes a day ahead of India’s planned rollout of the vaccine, dubbed as the world’s biggest vaccination drive ever.
Nepal has been looking for at least 12 million doses of Covid-19 vaccine to inoculate 20 percent (or 6 million) of its population and is planning to roll out vaccines first for health-care workers and volunteers, people above 60 years and other frontline workers.
As of Friday, Nepal has reported 266,816 Covid-19 cases and 1,948 deaths.




 8.67°C Kathmandu
8.67°C Kathmandu















