Health
Private firm seeks permit to import, sell Indian vaccine
It has told officials it can import 500,000 doses of Covaxin in three months.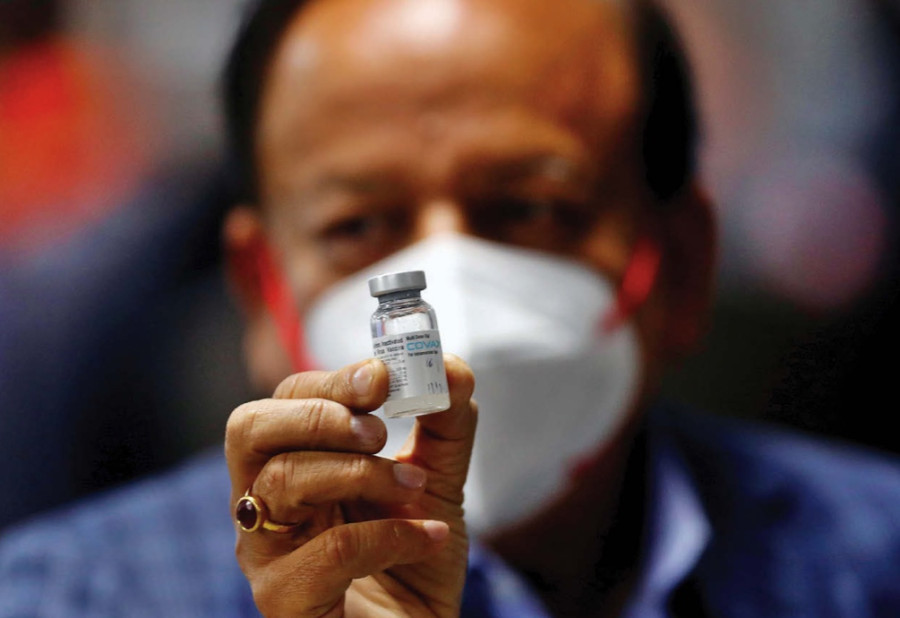
Arjun Poudel
Amid uncertainty surrounding the supply of coronavirus jabs in the country, a private firm has sought permission to import 500,000 doses of a Covid-19 vaccine developed and manufactured in India.
Covaxin, developed by Bharat Biotech received emergency use authorisation in Nepal on March 19, making it the third Covid-19 jab to be approved for use in the country.
“We have submitted our application on Monday to import Covaxin,” Surendran Nair, director at Emerald Pharma, told the Post. “We have applied to import 500,000 doses.”
Nepal is the third country in the world to provide emergency use authorisation to Covaxin after India and Zimbabwe, according to Reuters.
Nair said that the Department of Drug Administration, the national drug regulatory authority, has assured Emerald that it will make a call on the application at the earliest.
“We have applied to import vaccines in several batches as the manufacturing company too can’t provide all the doses at once,” he added. “The Indian government has also directed vaccine-manufacturing companies to prioritise supply within India.”
According to a source at the Department of Drug Administration, Emerald Pharma has sought permission to sell the two-dose jab at Rs 2,600 per dose to the members of the public.
“Yes a private company has submitted an application to import Covaxin,” an official at the department, told the Post, asking not to be named. “We will take a decision on the application after a meeting of senior officials.”
Bharat Biotech, along with two other manufacturers, had applied for emergency use authorisation for its vaccine on January 13. Of the three applications, the department had first granted emergency use authorisation to Oxford-AstraZeneca on January 15.
The AstraZeneca vaccine, manufactured by the Serum Institute of India under the name of Covishield brand, was then accordingly brought to the country. Nepal granted emergency use authorisation to BBIBP-CorV vaccine, developed by China’s Sinopharm on February 17.
China has provided 800,000 doses of BBIBP-CorV vaccine, which was brought to the country on Monday. Covaxin is India's homegrown and government-backed vaccine. It has an efficacy rate of 81 percent, preliminary data from its phase 3 trial shows, according to the BBC.
India's regulators granted the vaccine emergency approval in January while the third phase of the trial was still under way, sparking scepticism and questions from experts.
Nepal launched its vaccination drive on January 27 with one million doses of Covishield it received from India under grant assistance.
Of the 2 million doses for which Nepal signed a deal with the Serum Institute, one million doses have already arrived and the remaining doses are expected to arrive soon. Nepal also received 348,000 doses of vaccine under the Covax facility.
So far, over 1,790,000 people have taken the first shot in the first and second phases of the vaccination campaign. During the first phase of the vaccination drive (between January 27 and March 5), 438,879 people were vaccinated while over 1.25 million people took the first shot of the jabs in the second phase from March 7 to March 15.
Meanwhile, the Ministry of Health and Population said that it has not yet decided who will receive the vaccine provided by China under grant assistance.
The vaccine will most likely be provided to people serving in the organised sector such as school teachers and those serving in public transportation.
“A meeting of the experts panel will decide who will get the vaccine,” Dr Jageshwor Gautam, spokesperson for the Health Ministry, told the Post. “We are still discussing.”
Nepal Airlines Corporation’s plane flew to Beijing on Sunday to bring in the first consignment of China-made vaccine on Monday morning.
It is reported that the vaccine can be administered to people between 18 to 60 years old.
The Health Ministry’s effort to purchase 5 million doses of Covishield could not succeed, as officials said that the vaccine manufacturing company did not show interest in selling the jabs directly to the Nepal government at the price it sold earlier. The government had purchased 2 million doses at $ 4 per dose earlier.




 9.89°C Kathmandu
9.89°C Kathmandu















