Health
Vaccine hesitancy poses challenge for Nepal’s campaign against Covid-19
Tackling misinformation is as important as ensuring efficient supply and equitable access, according to WHO.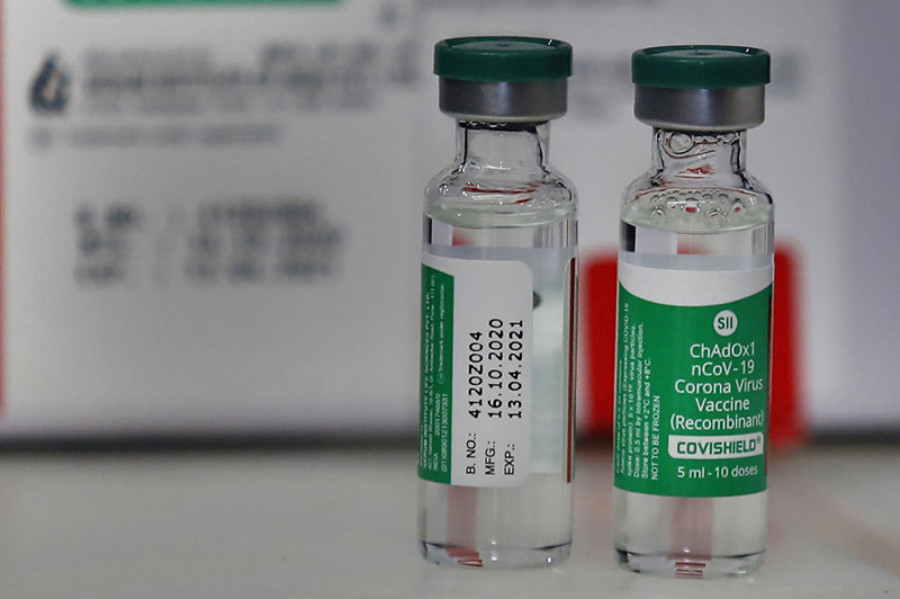
Arjun Poudel
Several health care providers, including doctors deployed on the frontlines have asked Dr Kiran Pandey, a consultant physician, whether they should take the Covid-19 vaccine or not.
The health workers doubt that the vaccines meet quality standards and fear side effects even as authorities prepare to roll out the Covishield vaccine, provided under an Indian grant, from Wednesday.
“I am surprised by the questions health workers, including doctors from my own circle, have asked about the vaccine,” Pandey told the Post. “I found a kind of hesitancy among healthcare workers—something that had not been seen in Nepal before.”
As Covid-19 vaccines are unconventional as were developed in a short period. That might have raised concerns among health workers, experts say.
“We finally have the vaccine. No one had expected that it would be available so soon,” added Pandey. “When people get something unexpected, it is difficult for them to accept it.”
In 2019, the World Health Organization declared vaccine hesitancy as one of the top ten global health threats. Doctors say that hesitancy for Covid-19 vaccines may increase, when it is rolled out to the masses.
“People are inevitably exposed to misinformation, rumours and false conspiracy theories, which may erode their confidence in vaccination,” reads the WHO’s statement. “It is important to build trust in the Covid-19 vaccines before people form an opinion against them.”
The UN health agency said that even after overcoming the imminent challenges of sufficient supply, efficient rollout and equitable access, a range of well-designed programme strategies will be needed to drive acceptance and uptake of the vaccine.
“Communicating consistently, transparently, empathetically and proactively about the uncertainty, risks and vaccine availability will contribute to building trust,” the WHO said.
Along with the vaccine hesitancy, managing possible adverse events following immunisation (AEFI) will be another challenge for authorities during mass immunisation drives.
“As only frontline workers will be inoculated under the first phase, authorities are, vaccine hesitancy may not be an immediate problem ,” Dr Bikash Lamichhane, former director at the Child Health Division, told the Post. “But authorities should be prepared for any untoward situation when the vaccine is rolled out for the masses.”
People are excited over news of availability of the vaccine, but any untoward incidents after the immunisation will have a negative impact on acceptability. Doctors say no drug or vaccine is hundred percent safe and vaccines against coronavirus may also cause some side effects in some people.
As the people and media are highly sensitive to news on vaccines, people will come to know of any adverse events, experts say. They believe that such events may impact the acceptance of the vaccine.
“It will be very difficult to convince the people, if they lose their confidence in the vaccines,” added Lamichhane. “We had to struggle to convince people to take the drug for elephantiasis.”
Even 12 years after the mass drug administration programme was launched, the prevalence of lymphatic filariasis has not been contained in some districts as people refused to take the medicines after some side effects were seen in some people.
“Preparation and awareness play an important role in raising confidence in the vaccine, and quash rumors,” Dr Sagar Rajbhandari, director at the Sukraraj Tropical and Infectious Disease Hospital, told the Post. “Even if some reactions are seen after immunisation, we should tell people to take it easily, as it shows the jab is working for them.”
Meanwhile, officials at the Ministry of Health and Population said final preparations are underway to roll out the vaccine for frontline workers. India has provided 1 million doses of the Covishield vaccine developed by the University of Oxford and pharmaceutical giant AstraZeneca and produced by Serum Institute of India.
“We have almost completed all necessary preparations to roll out the vaccine,” Dr Bikash Devkota, chief of Quality Standard and Regulation Division, told the Post. “As we have planned to offer the shots at health facilities, we hope we won’t encounter any problems during the first phase.”
The one million jabs are sufficient to inoculate three percent of the population deployed on the Covid-19 frontlines.
Devkota said that the ministry is quite aware about strategies to address vaccine hesitancy and tackle misinformation on side effects and has been working to deal with them accordingly.




 8.93°C Kathmandu
8.93°C Kathmandu