Health
Nepal lacks equipment to test harmful substances in syrupy medicines
Admission comes amid quality concerns of medicines in supply as cough syrups are linked to deaths in two countries.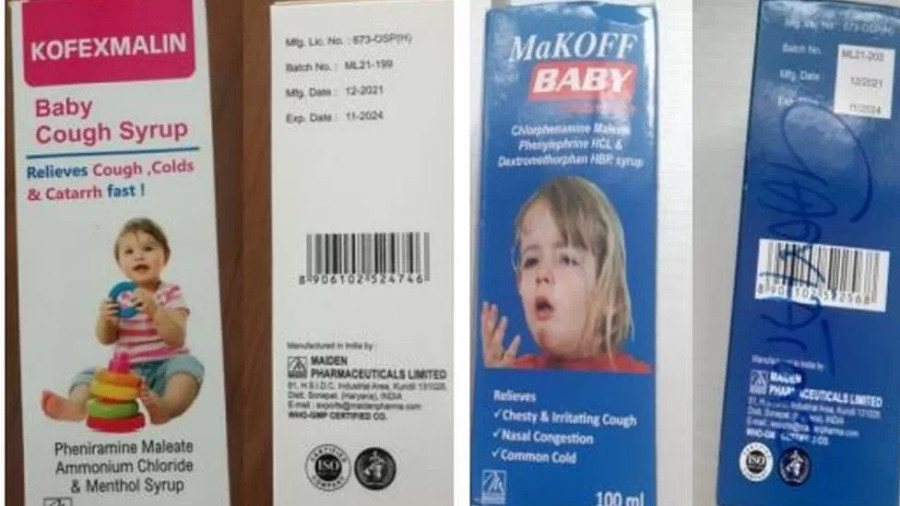
Arjun Poudel
Amid growing concerns over the quality of medicines especially cough syrups after scores of children died in the Gambia and Indonesia in recent months, the National Medicine Laboratory has said it lacks the equipment to test harmful substances in syrupy drugs.
“We don’t have the equipment to carry out tests for harmful substances in syrupy drugs,” Pan Bahadur Kshetry, acting director at the laboratory, told the Post. “Our attention has been drawn to the deaths of children from harmful substances in such drugs in some countries.”
Health authorities in Indonesia said the number of child deaths from kidney failure or damage linked to harmful substances found in syrupy medicines has risen to 195.
Indonesia has witnessed a spike in cases of acute kidney injury in children since August, and over 320 cases of the said problems have been recorded throughout the country.
Similarly, the deaths of 70 children in Gambia, the West African country, in July, August, and September, have been blamed on substandard products.
The World Health Organisation issued a global alert about four cough syrups manufactured by an Indian Pharmaceutical Company, Maiden Pharmaceuticals, that could have links to the deaths of children in the Gambia.
The Department of Drug Administration had alerted drug inspectors to step up surveillance to prevent the import and sales of cough syrups manufactured by Maiden after a global alert by the UN health body.
The UN health agency identified four medicines manufactured by the Indian company—Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup, and Magrip N Cold Syrup—as substandard. These medicines could be responsible for the deaths. The WHO said that the company had failed to guarantee the safety of the products.
The Indian government is also investigating the situation, according to media reports.
Diethylene glycol and ethylene glycol are used as antifreeze, brake fluids, and in other industrial applications, but also as a cheaper alternative in some pharmaceutical products to glycerine, a solvent or thickening agent in many cough syrups, according to a Reuters report.
Concerns about the quality of drugs grow as Nepal and India share hundreds of kilometers of porous border, through which thousands of people cross over to each other’s territories every day. Small traders smuggle various goods including medicines into Nepal taking undue advantage of the unguarded areas of the border.
The WHO has called for increased surveillance and diligence within the supply chains of countries and regions likely to be affected by these products. It has also advised increased surveillance of the informal/unregulated market.
Around 60 percent of the total pharmaceutical drugs sold in Nepal come from India.
Officials concede that risk is not only in imported drugs, but also in the drugs manufactured within the country.
“Accidents can happen at any level and no one can deny human error,” said Kshetry. “We need to strengthen our regulatory mechanism including our laboratory resources.”
Officials at the Department of Drug Administration concede that the government cannot examine the quality of each and every drug, and the drug manufacturers themselves have to ensure the safety of their products.
“Drug manufacturing companies themselves check the quality of every batch of drugs in their internal laboratories and submit reports to the drug regulatory body,” said Biplab Adhikari, general secretary of the Association of Pharmaceutical Producers of Nepal. “For products of new formulation, we have to carry out tests from an independent lab accredited by the government agency. The National Medicine Laboratory also performs tests to ensure the quality of medicines.”




 9.7°C Kathmandu
9.7°C Kathmandu















