Money
Vaidyakhana to adopt good manufacturing practices
Venerable herbal medicine producer Singha Durbar Vaidyakhana Bikas Samiti has moved to adopt good manufacturing practices (GMP) after receiving the green signal from the Department of Drug Administration.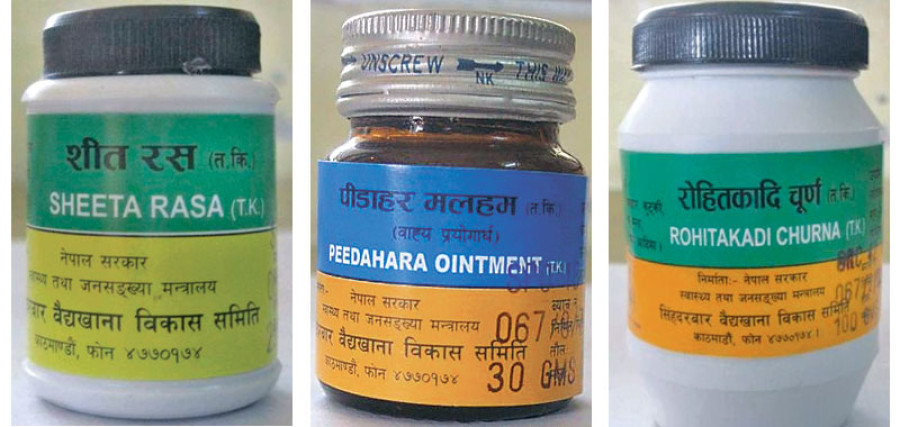
Prahlad Rijal
Venerable herbal medicine producer Singha Durbar Vaidyakhana Bikas Samiti has moved to adopt good manufacturing practices (GMP) after receiving the green signal from the Department of Drug Administration.
The department has approved the state-owned herbal medicine company’s application to build a GMP certified production house under WHO guidelines, which include upgrading its production equipment, lab and building.
The company has started the process to invite bids for the construction of the required facilities. According to the company, the design of the proposed new production facility has been approved.
“This will enable the company to produce drugs under WHO standards,” said Babu Raj Amatya, senior Ayurvedic physician at the department. “The medicines produced by the company will be certified under the WHO standard once it has all the required facilities.”
According to Amatya, the company has not received the final approval to produce and sell medicines under GMP guidelines. “Going into the GMP process does not mean they can produce and sell products with WHO certified labels. We will assess the medicines and certify them after they are produced in an upgraded facility.” Singha Durbar Vaidyakhana Bikas Samiti, the oldest Ayurvedic company in Nepal, produces herbal medicines like Silajit, sugar-free Chyawanprash, Sharpagandha and others targeting the domestic market. It makes more than 140 types of medicines.
The objectives of GMP guidelines are to provide guiding principles for assessing the quality in relation to the safety of herbal medicines, with specific reference to contaminants and residues, according to the WHO. It also ensures procedures for controlling the quality of finished herbal products before they reach the market. The WHO says that there is a need to regulate the herbal industry because as a consequence of market globalisation, many of the medicines used in phytotherapeutic systems do not come from the country of origin but from third countries.
Nepal’s oldest Ayurvedic company had produced and sold herbal medicines worth Rs29.7 million last year. It has set a target to produce medicine worth Rs40 million this year amid growing demand.
Meanwhile, the upgradation plan is likely to face delays due to lack of funds. The construction cost of the proposed facility has been estimated at Rs170 million, but the Finance Ministry has allocated a budget of only Rs10 million. “However, we have requested the ministry to increase the budget,” said Rajendra Kumar Giri, managing director of the company. “We have planned to send our products to markets abroad within five years, but budget constraints have hampered our plan,” he said, adding that the government should accord priority to the production of high-value herbal medicines.
According to Giri, after the fulfilment of the GMP requirement, they will be able to produce and export standardised medicines abroad. Nepal has an abundance of herbal resources but lacks quality control mechanisms and WHO mandated facilities. Bhaskar Herbaceuticals is the only company currently producing Ayurvedic medicines in Nepal under WHO guidelines.




 20.12°C Kathmandu
20.12°C Kathmandu