Editorial
Killer medicines
A well-equipped and big enough drug-testing laboratory will be worth every rupee of investment.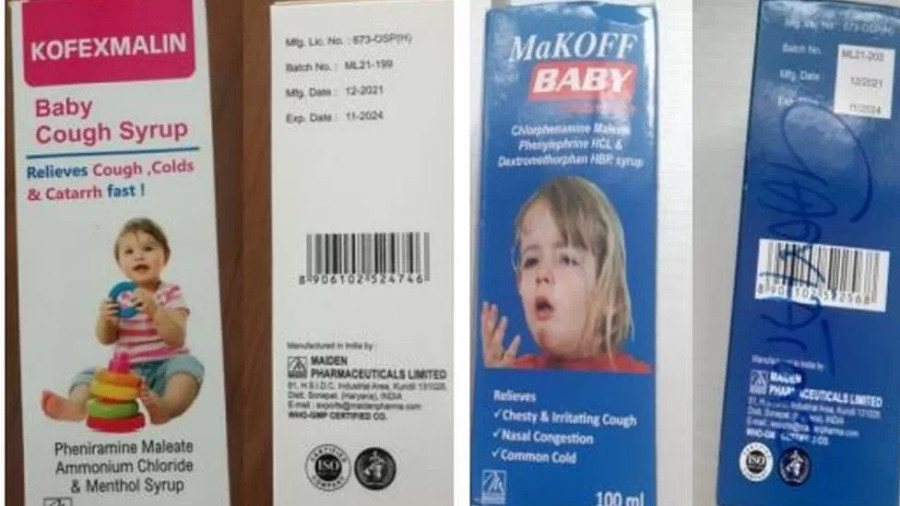
The deaths of hundreds of children in The Gambia and Indonesia, which have been linked to India-made syrupy drugs, have rocked the world, prompting the World Health Organization to issue a warning. As of now, The Gambia has reported around 70 drugs-linked child deaths while Indonesia has reported over 200 such deaths. The syrups—Promethazine Oral Solution, Kofexmalin Baby Cough Syrup, Makoff Baby Cough Syrup and Magrip N Cold Syrup—according to the WHO, have been linked to these deaths. The syrups contain what the WHO calls “unacceptable amounts” of diethylene glycol and ethylene glycol, which in turn are “potentially linked with acute kidney injuries". Rather alarmingly, in Indonesia, five locally made products had high levels of ethylene glycol, pointing to the greater risks of unregulated pharmaceutical markets everywhere, not the least in monitoring-lite Nepal.
While The Gambia and Indonesia have banned the import and sale of the said syrups, there is a risk that the syrups could have been distributed to other countries through informal markets. After all, we live in a globalised world which often facilitates uninterrupted flow of materials, including medicines. The fact that Nepal shares an open border with India should horrify Nepali authorities and compel them to work tirelessly to ensure that the syrups do not enter Nepal through formal or informal channels. To their credit, they are reported to have alerted drug inspectors to step up surveillance to prevent the import and sale of drugs manufactured by Maiden, the Indian manufacturer of the lethal syrups. That is not enough though. Without good labs to test the safety standards of the medicines—and not just from Maiden—Nepal will continue to be at high-risk for mass illnesses, even deaths.
The lethargy with which Nepali authorities run public institutions often astounds even Nepalis, as we saw during the Covid-19 pandemic and the ongoing dengue outbreak. No one should be surprised if they continue to feign helplessness if Nepal faces a grave crisis like The Gambia and Indonesia. But how long can our officials operate with systemic negligence, failing to acquire the crucial equipment required to test for harmful substances in syrupy drugs even as a health crisis lurks? If our record at handling such crises have been less than exemplary, the only way forward is to continue getting better. That, sadly, is not the case even when the country is staring at a grave crisis. Although we have been lucky so far, there is no room for complacency. The authorities should acknowledge that an informal drug market operates in Nepal, right under their noses, and that is where they should look, apart from formal markets, to ensure that the lethal drugs do not circulate in Nepal. But this is also not just a matter of checking the quality of syrupy medicines. Many Indian and Nepali drug manufacturers are reportedly cutting corners or failing to take adequate measures to ensure the safety of their medicines. Irrespective of the cost involved, building a well-equipped and big enough drug-testing laboratory will be worth every rupee of investment when what is at stake is the health and wellbeing of potentially millions of Nepalis.




 9.89°C Kathmandu
9.89°C Kathmandu














