Health
Nepal drugs regulator halts sales of Indian antibiotic injection Biotax 1gm
The antibiotic, vital for treating multiple infections, was found substandard in lab test.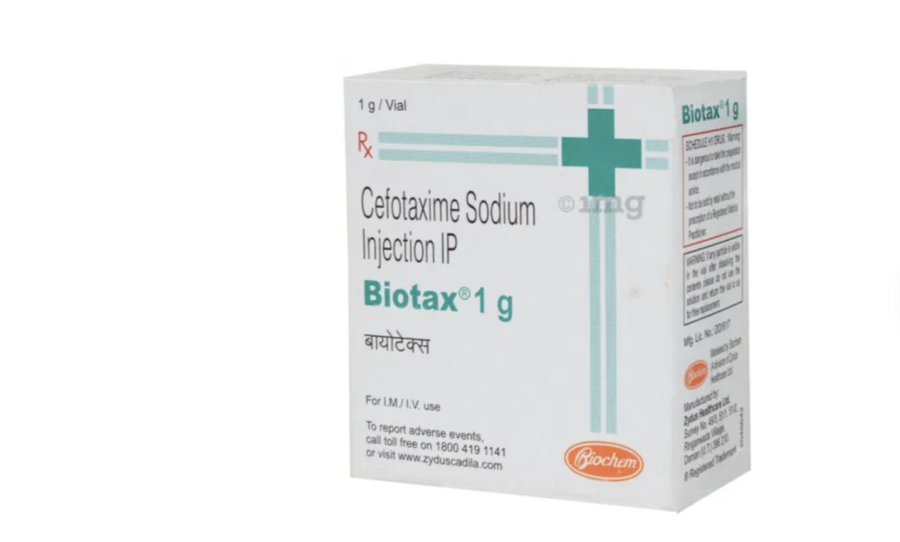
Post Report
The Department of Drug Administration has suspended the sales and distribution of Biotax 1gm, an antibiotic injection manufactured by an Indian firm, citing serious health risks.
According to the department, the national drug regulatory body, laboratory testing of the ‘Biotax 1gm’ batch F300460, manufactured by Indian pharma company Zydus Healthcare Ltd, revealed that the drug was not in compliance with the production specifications.
“We have directed the manufacturing company, importers and distributors to immediately suspend sales, import and distribution of the said medicine, until further notice,” said Pramod KC, spokesperson at the department. “Some serious issues have been detected in the said antibiotic. Decisions about further actions will be taken once the investigation is completed.”
Biotax 1gm injection is an antibiotic medicine used to treat bacterial infections, including those of the brain, lungs, ear, urinary tract, skin, soft tissues, bones and joints, blood, and heart. It is also used to prevent infections during surgery.
According to the department, which carried out the testing in its own laboratory, BIOTAX-1gm batch F300460 is not safe for use and could risk patients’ lives.
KC said that the decision to suspend the sales of the injection was made to ensure patient safety and would not affect the treatment, as other drugs with the same composition are available in the market.
“Injection of the same composition manufactured by other companies are available in the market,” said KC. “The suspension of sales will not affect the treatment of patients.”
Suspending and recalling substandard drugs from the market is routine work and part of the department's risk reduction measures. Officials said that several medicines found to be substandard have also been suspended from sales and distribution or recalled in the past.
However, it is not known if all those substandard drugs were successfully recalled or some were sold to patients, as the department does not have the capacity to ensure timely recess of all substandard medicines.
The regulatory agency regularly collects drugs through random sampling methods from pharmacies across the country and tests them in its own laboratory to ensure compliance with quality standards.
DDA officials admit that due to understaffing, they cannot always monitor if all those drugs have been recalled or not.
The department publishes a notice on its website asking respective companies to recall those drugs as soon as possible.
However, most of the time, those substandard drugs have already been sold out by the time the notices are published, as it takes months, mainly due to understaffing, for the department to conduct tests in its laboratory.




 13.12°C Kathmandu
13.12°C Kathmandu