Health
Children’s antibiotic drug being sold in the market for the past 20 months found to be substandard
Department of Drug Administration has directed the concerned manufacturing company to recall its substandard drug.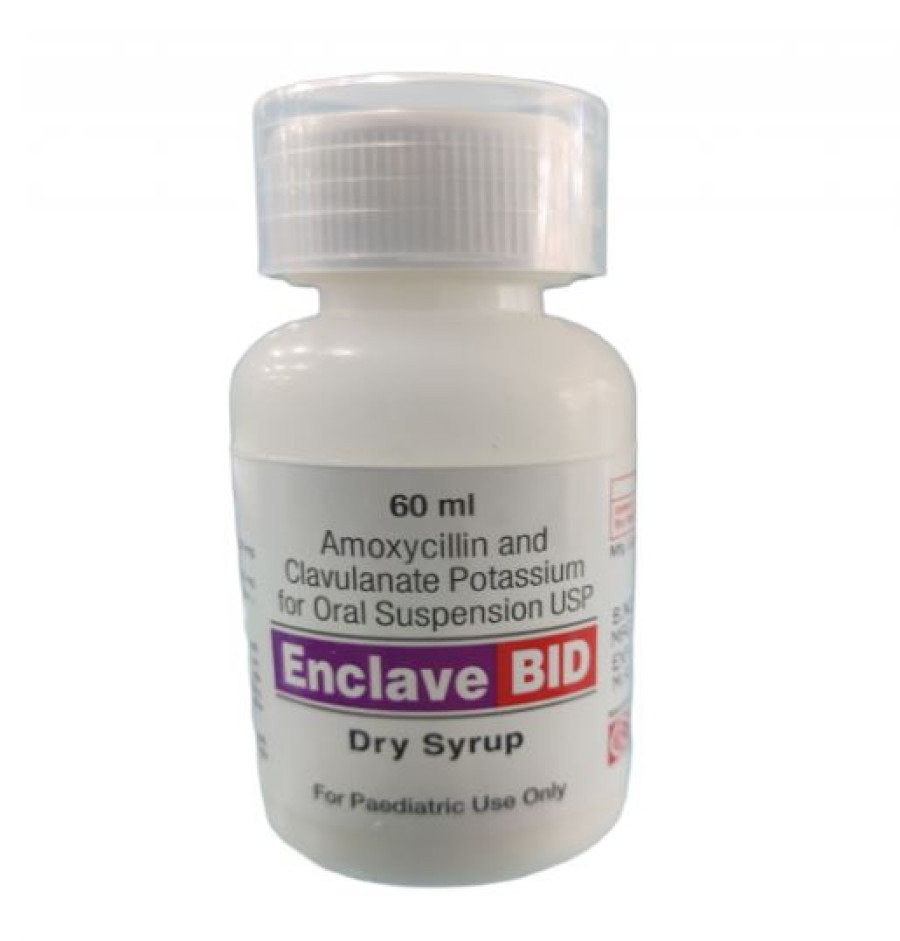
Arjun Poudel
An antibiotic being sold in the market since January 2022 has been found to be substandard in a laboratory test.
The Department of Drug Administration, which recently carried out lab testing on Enclave Bid, a paediatric antibiotic manufactured by Curex Pharmaceuticals Pvt Ltd, found that the medicine didn’t meet the standards.
“We have directed the manufacturing company to recall all the medicine of the DEB-168 batch from the market and inform us,” said Narayan Dhakal, director general of the department. “This medicine does not cure the ailment, prolonged the duration of the ailment and if used, another antibiotic is needed for cure.”
Curex Pharmaceuticals is a Kavre-based national drug manufacturer. The department, which is the national regulatory body of pharmaceuticals as well as Ayurvedic medicines in the country, said that the amount of the content in the medicine has been degraded to 64 percent.
Experts say if the antibiotic was given to a child for the treatment of an infection, his/her ailment would have exacerbated.
When asked why the lab testing of the medicine was carried out at the time of its expiration and when the medicine might have already sold out in the market, officials conceded that their drug inspectors could not collect the samples of the drug on time and the laboratory also took time to carry out testing.
“Had we carried out the lab testing on time, we could have recalled the substandard drug months ago from the market,” said Dhakal. “That could have prevented ailments from exacerbating, helped patients to get cured earlier and saved unnecessary expenses for the patients.”
The department carries out lab testing on samples of medicines collected randomly by its drug inspectors from the market. And most of the time, when the department unveils its lab reports and recalls faulty drugs, most of the substandard drugs will already have been sold.
The department’s officials could not say if the recalled substandard drugs are still on the market or not. They reasoned that the department doesn't have the capacity to monitor each and every pharmacy.
Doctors say substandard antibiotics are among the reasons for the high burden of antimicrobial resistance in Nepal, which ultimately leads to the irrational use of several types of antibiotics for the treatment of infections.
They warned that antibiotics, which have saved millions of lives across the world, could soon become ineffective due to the high resistance rate caused by their irrational use.
In a study carried out in Nepal on humans, animals and environment samples, antimicrobial resistance genes were detected in 81 percent of the total samples on which antimicrobial resistance tests were carried out.




 16.12°C Kathmandu
16.12°C Kathmandu