Columns
Death by medicine
Indian pharmas need a thorough revamp to protect the lives of people in developing countries.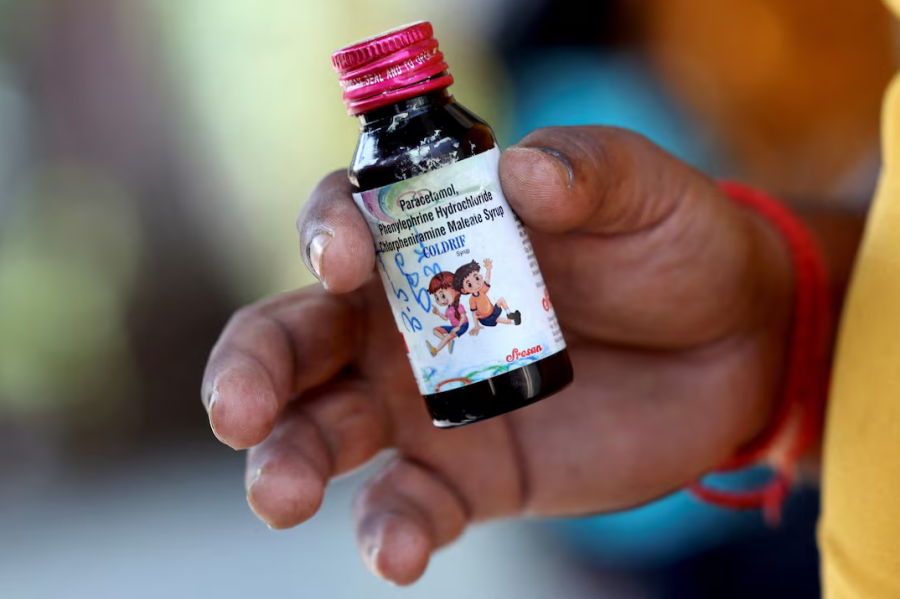
Kashif Islam
In a shocking development, nearly 25 young children died after consuming adulterated cough syrup in the Indian state of Madhya Pradesh. The cough syrup was found to contain toxic levels of diethylene glycol, an industrial solvent which is occasionally used to adulterate syrups.
The deaths started getting noticed when children started taking ill in mid-September after consuming a brand prescribed by a local doctor. The state government was slow to react when the deaths began getting reported. The testing of samples took too long, and even before the results came in, the State health minister declared the cough syrup safe.
Only once, Tamil Nadu, the state where the toxic syrup had been manufactured, warned that it had found toxic levels of the chemical, did the Madhya Pradesh government swing into action. The state drug authority raided pharmacists and stockists, and the health ministry suspended various officials, including the doctor who prescribed the syrup. Pharmacies stopped dispensing all cough syrups, fearing government raids. But by then, 25 precious lives had been lost.
A systemic problem
It would be a mistake to dismiss the tragic event as an isolated occurrence. In fact, a disaster like this was waiting to happen. For years, doctors and activists have been complaining of sub-standard and spurious drugs in the Indian market. From fake anti-cancer drugs to starch tablets disguised as antibiotics, reports on such incidents are rife. There are no hard figures, given inadequate testing and a lack of transparency on inspection results.
In 2024, the Indian drug control authority released a list of 50 popular medicines made by large pharmaceutical companies that failed the standard quality testing. Many of the companies claimed that the batches tested were spurious and never made by them. Action against errant manufacturers is rare.
A highly profitable industry
Drug manufacture has become a highly competitive and profitable industry. Compared to other industries, setting up a small drug unit requires less capital investment. Many of the smaller companies are simply mixing and packaging units, sourcing the active pharmaceutical ingredients, often from China, and packaging them into pills, capsules and syrups.
There are thousands of small-scale pharmaceutical units just like the cough syrup maker who operated out of a small building and made several dozen products. Even with established companies, there is an intricate web of subcontracting where larger companies find it profitable to outsource their manufactured products to small companies.
It is usually believed that competition improves outcomes for consumers, but not in the pharmaceutical industry. Since public advertising is not allowed, the companies spend a lot on pushing their products to doctors and frequently offer expensive benefits to prescribing doctors.
Lax regulatory oversight
While every pharmaceutical unit is covered by a host of regulatory requirements, including licensing and quality control, few of these are achieved in actual practice. Food and drug control is mainly the ambit of the states, and a few states have the required drug inspectors and testing capacity. In Madhya Pradesh itself, 80 drug inspectors were serving a population of 90 million and the three testing labs were said to have a year-long backlog of testing.
The testing that does happen is not adequate. The inspection reports are not made public and very few regulatory actions result from these. Reports indicate a close nexus between the drug inspectors and the pharma companies. Many former inspectors have become independent consultants helping the industry to get through inspections and regulatory formalities.
The result of the lax regulatory environment is that patients cannot trust lesser-known generic variants of branded drugs for fear that they may be substandard. Those who can afford do not use drugs supplied by government hospitals. This has allowed established companies to hike their prices compared to their lesser-known variants.
Global repercussions
The problem of spurious and sub-standard drugs is compounded by the fact that India is also one of the largest exporters of generic medicines. In fiscal year 2025, Indian pharmaceutical exports rose to a record USD 30 billion. The United States was the top destination, followed by the UK and France. Indian companies export not only to the US and Europe but also to Africa and South Asia.
While there are stringent quality requirements in the US and Europe, ensuring only the best quality drugs made by reputed companies are supplied, this is not the case in countries with less stringent requirements. Many developing nations rely on the testing done in the exporting country.
As a matter of fact, tainted cough syrups from India have been implicated several times in the past. In 2022-2023, more than 100 children died in Gambia, Cameroon and Uzbekistan after consuming cough syrups imported from India. At the time, this was disputed by India, though the WHO attributed the deaths to India-made cough syrups. The company behind the tainted cough syrup was accused of bribing officials to replace the samples and was never prosecuted in India.
The story of the dying children will be forgotten in some time, but the Indian pharmaceutical sector needs a thorough revamp to protect the lives of ordinary Indians and people in developing countries. Knee-jerk reactions such as suspending officials and banning specific brands are unlikely to fix the problems of lax regulatory oversight and questionable manufacturing practices.




 9.7°C Kathmandu
9.7°C Kathmandu